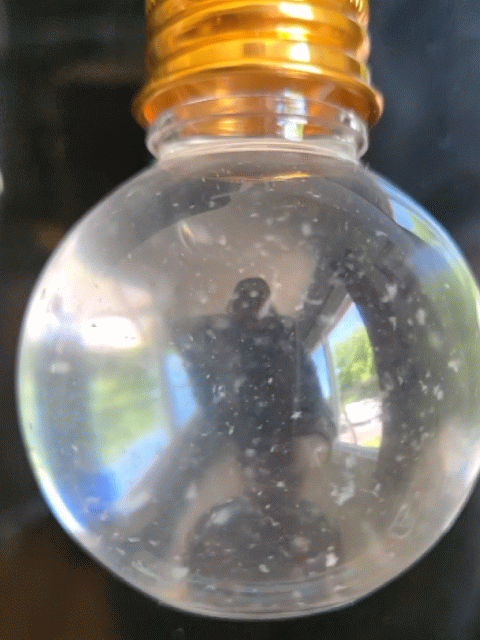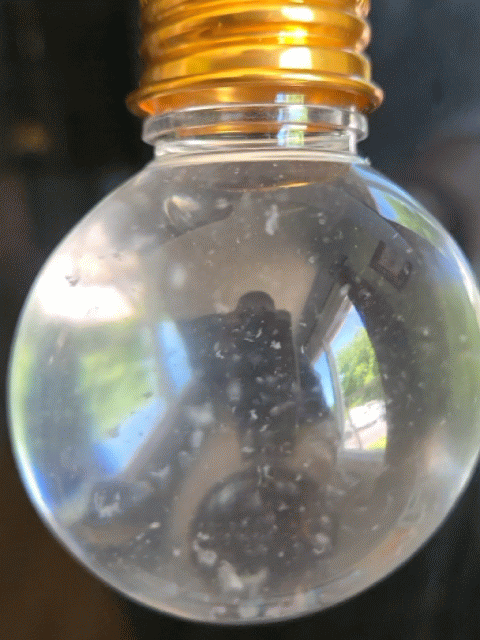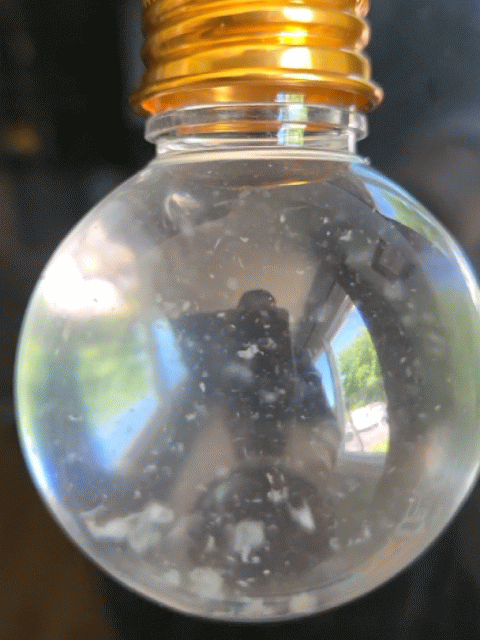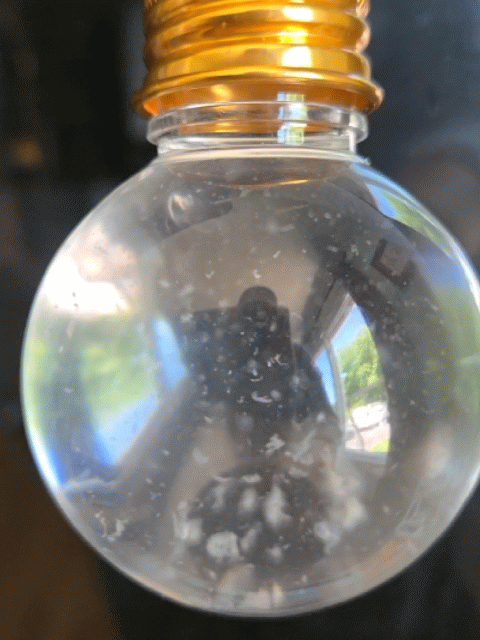Why I Like It When Experiments Don’t Quite Go As Planned During a Class or Workshop
I recently did a fun workshop with eight curious middle schoolers and their parents where we made Strawberry DNA Snow Globes! We talked about biology, chemistry, and using biology as technology. One of the groups dove into a discussion about the proteins involved in our immune system and genetic engineering, including booting up artificial genomes in recipient cells! Did I mention this was a group of 10-12 year olds, middle school students? I loved it!
Everyone got to make their own snow globe with real strawberry DNA inside! However, this time, the DNA didn't act quite as expected. In this experiment, the DNA usually floats to the top of the alcohol layer quickly, but this time it took much longer or it just hung out where the water (red) and alcohol layers meet (the interphase). The interphase is where DNA hangs out during a regular DNA extraction; however, for a snow-like appearance, we want it to float to the top of the alcohol and be very dehydrated, appearing white and crispy, so it can break into smaller chunks and imitate snow in the snow globe.
Strawberry DNA precipitating (clumping) and rising to the top of the alcohol layer
Why This Was Actually Great!
Being at the BioMakerSpace (a community lab) when this experiment did not work as expected was perfect! Because we were in a lab, we could not only discuss and ask questions, but also try different things on the fly and make the experiment work. This meant we could still finish the project and learn even more!
Usually, in a simple strawberry DNA extraction in a test tube, the DNA is spooled onto a toothpick or a wooden dowel from the interphase. DNA extracted this way looks like slimy strings hanging from the toothpick, because the DNA is still holding on to some water molecules. It works anywhere and is more tolerant of errors or substitutions. But most bio-related science experiments are much more sensitive to changes. If something goes wrong, it can be challenging to fix without access to additional reagents and equipment that are typically available in a real lab. And when experiments fail or don’t work as expected, without the ability to troubleshoot, make it work, and learn from the experience, participants can get disappointed, frustrated, and lose interest in science after repeated setbacks. When talking to parents, especially in the homeschool community, where access to a lab is not easily available, tricky experiments that don’t work as expected are the number one reported to me reason, for losing interest in science and avoiding hands-on activities for both the student and the homeschooling parent.
The Soap Mystery!
I think we figured out what happened. I've done this experiment many times before with great results. But this time, I used a different soap! I only had one tube of the old soap, and guess what... only one snow globe worked as expected (with the DNA floating to the top quickly, looking snow-white and crispy). This made us think it was the soap.
Both soaps had a special ingredient called EDTA (which helps protect DNA during the experiment), but they had different types of EDTA and some other differences in ingredients. So, it seems the type of soap really made a difference for this kind of DNA extraction experiment. While both EDTA types work well for DNA extraction, it looks like for the snow globes, the soap with tetrasodium EDTA works better. Since the EDTA type is not the only difference between the two soaps, we can't say that the EDTA type alone is the cause for the different outcome of our experiment; however, we can guess that the different composition of the new soap was what caused the experiment not to work as expected. I said “guess” because we made an observation and based on it, we can hypothesize (make an informed guess), what the cause was. To conclude that the different soap really was the cause (to confirm our hypothesis) we would have to do side-by-side experiments with both soaps and collect new evidence to confirm or reject our hypothesis. Let me know if you want to do this with me 👩🔬 because the scientist in me has to know for sure 😂
What We All Learned (Bonus Learning):
Even though the experiment almost failed, everyone went home with strawberry DNA in their snow globe and we learned even more than I had planned:
Sometimes science experiments don't work the first time. Biology and chemistry can be tricky, and in a biology research lab, experiments rarely work as expected the first time we do them!
Changing just one thing (like the soap) can make a big difference.
We can often adjust experiments as we go, and we did, or repeat them another day with adjustments! Everyone still got DNA "snow" in their globes.
We used a high-speed centrifuge to spin the tubes with DNA in the water-alcohol solution at a speed that generated 15,100 times the gravity force and spun the tubes for 1 minute at that high speed to help pull down the DNA clumps to the bottom of the tubes and separate them from the liquid, so we can transfer the DNA to the snow globes. For comparison, a Formula One driver and a roller coaster rider experience up to 5-6 times the gravity force in short bursts.
We added an extra filtration step to ensure everyone's snow globe was clear and beautiful.
I also learned something new: not all soaps work well for this DNA extraction method to make DNA snow globes. I love learning something new with every experiment! That's one of the best parts of being a scientist.
A mini snow globe with swirling strawberry DNA as the "snow"